Which element has the following ground state electron configuration? This question opens up a realm of inquiry into the fundamental properties of elements and their behavior. Ground state electron configuration plays a pivotal role in determining an element’s chemical and physical characteristics, providing a roadmap to understanding its position within the periodic table and its potential applications.
Delving into the intricacies of ground state electron configuration, we embark on a journey that unveils the secrets of atomic structure and its profound impact on the world around us.
Ground State Electron Configuration
Ground state electron configuration refers to the arrangement of electrons in the lowest energy state of an atom or ion. It is crucial in determining the chemical and physical properties of an element.
Significance of Ground State Electron Configuration, Which element has the following ground state electron configuration
- Predicts the reactivity of an element
- Determines the element’s position in the periodic table
- Provides insights into the bonding behavior of an element
Element Identification
Ground state electron configuration can be used to identify elements. Elements with similar electron configurations exhibit similar chemical properties and belong to the same group in the periodic table.
- Elements with the same number of valence electrons have similar reactivity.
- Elements with completely filled valence shells are noble gases and are highly unreactive.
Periodic Trends: Which Element Has The Following Ground State Electron Configuration

Ground state electron configuration influences various periodic trends, including:
- Chemical Reactivity:Elements with lower ionization energies are more reactive.
- Electronegativity:Elements with higher electronegativities have a stronger tendency to attract electrons.
- Atomic Radius:Elements with larger atomic radii are less reactive.
Quantum Mechanics
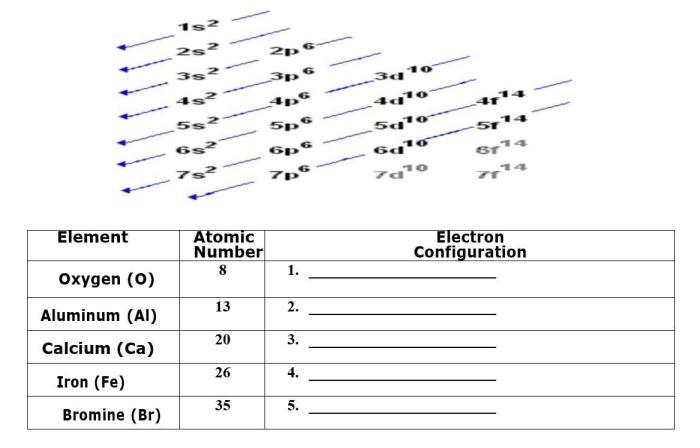
The quantum mechanical model of the atom explains the ground state electron configuration based on:
- Pauli Exclusion Principle:No two electrons can have the same set of quantum numbers.
- Hund’s Rule:Electrons occupy orbitals with the same spin before pairing.
These principles dictate the filling of atomic orbitals and determine the ground state electron configuration.
Applications
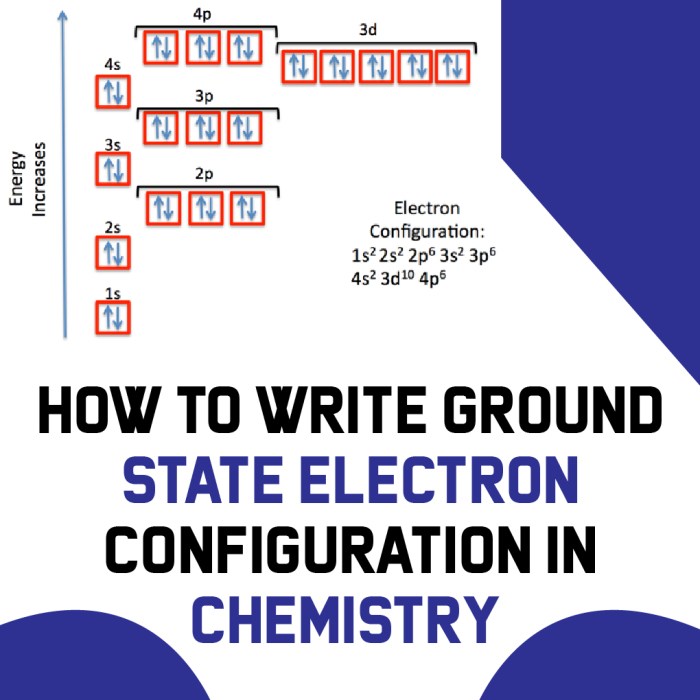
Understanding ground state electron configuration has practical applications in various fields:
- Chemistry:Predicting chemical reactivity, designing catalysts, and understanding bonding.
- Materials Science:Tailoring the properties of materials by controlling electron configurations.
- Astrophysics:Studying the composition of stars and galaxies based on their observed spectra.
FAQs
What is ground state electron configuration?
Ground state electron configuration refers to the arrangement of electrons in an atom’s lowest energy state, providing a blueprint for its chemical properties.
How does ground state electron configuration influence chemical reactivity?
Ground state electron configuration dictates the number of valence electrons, which play a crucial role in determining an element’s reactivity and bonding behavior.